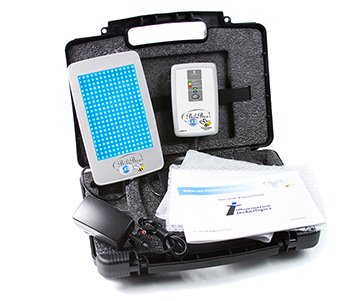
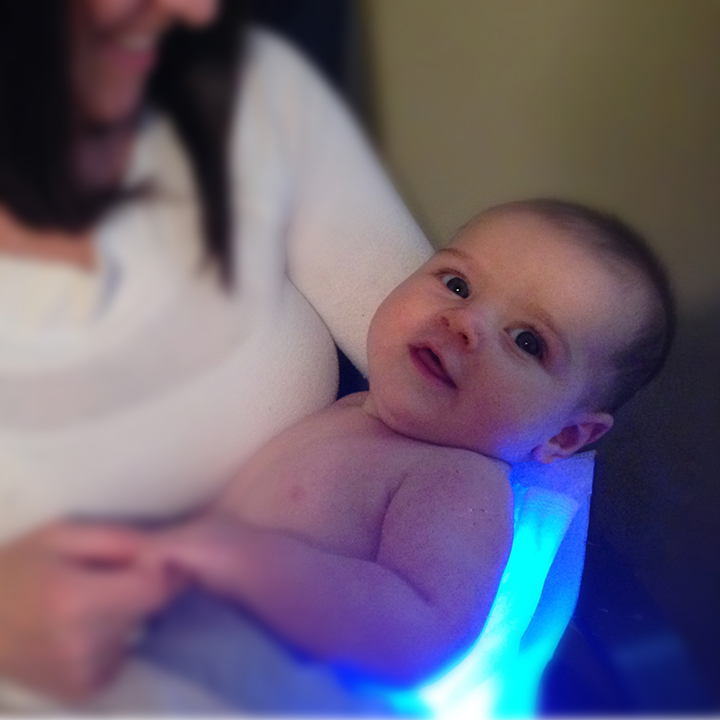
The BiliBee LED is a highly-portable device that utilizes an LED illuminator pad for the treatment of neonatal jaundice (hyperbilirubinemia).
LED | BiliTX | BiliSoft | BiliBed |
|
|---|---|---|---|---|
| FDA Approved |  |  |  |  |
| Promotes Mother/Baby Bonding |  |  |  |  |
| Lightweight |  |  |  |  |
| Battery Pack |  |  |  |  |
| Portable |  |  |  |  |
| LED Light Source |  |  |  |  |
| Maintenance Free |  |  |  |  |